To my Mother, to my Wife, and to all my Friends
This thesis demonstrates the usefulness of the exergy concept for analyzing systems which convert energy, material and/or information, e.g., a society or an industrial process. The first paper, Exergy - a Useful Concept within Resource Accounting, deals with the theory of exergy in a new and simple way. Exergy is applied to matter, is related to other thermodynamic potentials and to information theory.
Paper II, Exergy Conversion in the Swedish Society, presents, in terms of exergy, the conversions of energy and material resources in the Swedish society in 1980. Necessary concepts and conventions are introduced. Exergy losses in transformations of material resources and in conversions of various forms of energy into heat are described in some detail. Compared to the situation in 1975, described in Paper I, the change is mainly the increased use of nuclear power and the decreased use of fuel oil.
Paper III: Exergy Flows in Industrial Processes, describes the exergy flows in a pulp and paper mill and in a steel plant. Steam data are calculated on a micro-computer with the accuracy of ordinary steam tables. Also, a simple method for calculating the exergy of different substances is presented. For the purpose of comparison, the Swedish house heating system is described using the exergy concept. The energy and exergy prices of several common energy forms are also compared.
Thermoeconomics is an effective method of making technical systems efficient by finding the most economical solution within the limits of the technically possible. At the same time it may indicate how research and technical development should be directed. In Paper IV, Thermoeconomic Optimization of a Heat Pump System, a simple heat pump process is chosen as an example to illustrate the method. The physical treatment of the refrigerant and the optimization of the system is done with a specially developed computer program. The result shows, among other things, that the driving source should be made more efficient, thus saving both money and exergy.
Keywords: Exergy, resource conversion processes, applied thermodynamics, thermoeconomics, energy analysis, efficiency, processing industry, energy management, cost engineering.
This thesis treats the exergy concept and its applications to technical and societal systems. The purpose is to develop descriptive methods which are based on fundamental theory and to show their usefulness in different applications. The exergy concept often implies a new picture of energy and material conversion systems. The concept is not new but for a long time it was nearly forgotten. As is shown by the large number of publications during the last years it has now been rediscovered. One reason for this is the increasing interest in efficient energy techniques deriving from the problems related to energy use in the society. Exergy is a concept originating from an engineering problem. It is by now a firmly established concept in physics with well-defined relations to the information concept of information theory. That it takes time for a less familiar concept to be understood and accepted is shown by the fact that still to-day there is opposition against using exergy instead of energy in physical descriptions of resource conversions.
Hopefully, this thesis demonstrates the importance of the exergy concept for analyzing systems which convert energy, materials and/or information, e.g., a society or an industrial process. The large losses which are revealed in an exergy treatment of a process should be seen not as an insurmountable obstacle but as a challenge to achieve technical improvements. I hope that the exergy concept will reach further understanding and acceptance within science and education, research and development in the society.
I will here express my gratitude to all of those who have made this work possible. I will name some of them in particular. Karl-Erik Eriksson has always been of great help in many respects. Björn Eriksson and Kåre Olsson inspired the early tentative steps in this work. Sten Karlsson has, throughout the years, closely watched and criticized my work. Myron Tribus and Yehia El-Sayed guided me into my present field of research during my stay at Massachusetts Institute of Technology. The financial support of the Energy Research and Development Commission, the National Swedish Board for Technical Development, the Swedish Natural Science Research Council, and the Swedish Council for Planning and Coordination of Research is gratefully acknowledged. Finally, I am deeply grateful to my wife Kerstin for her help and patience.
1. Introduction
2.1. Definition
2.2. Why physical resource theory ?
2.2.1 Questions that arise within science
2.2.1.1. The origin of resources and their conversion in various systems
2.2.1.2. Various forms of resources
2.2.1.3. General thermodynamic and information-theoretical problems
2.2.1.4. A comment
2.2.2. Questions that arise within society
2.2.2.1. Resources within an economy
2.2.2.2. Resource use over time
2.2.2.3. Efficiency in resource use
2.3. Relations to other fields
3. Definition of the concept of exergy
4. Presentation of Papers I and II
4.1. Exergy conversion in the Swedish society in 1920
4.1.1. Introduction
4.1.2. Technical uses of natural resources
4.1.3. Agriculture
4.1.4. Forestry
4.1.5. Solid fuels
4.1.6. Liquid fuels
4.1.7. Electric power
4.2. Comparison of the presented societies
5. Presentation of Papers III and IV a-b
5.1. Paper III
5.2. Paper IV a-b
6. References
Appendix
Bibliography on Exergy (428k)
Papers:
I Wall, G., 1977, Exergy - a Useful Concept within Resource Accounting (310k)
II Wall, G., 1981 revised 1986, Exergy Conversion in the Swedish Society
III Wall, G., 1983 revised 1986, Exergy Flows in Industrial Processes.
IVa Wall, G., 1985, Thermoeconomic Optimization of a Heat Pump System.
IVb Wall, G., 1985, Thermoeconomic Optimization of a Heat Pump System- Computer Programs.
On the 18th of November, 1975, an article by Hannes Alfvén was published in the Swedish daily newspaper Svenska Dagbladet. The headline of the article was "Exergy report may create a new energy policy". The report that he was referring to was a preliminary draft of a paper (B. Eriksson, K.-E. Eriksson, K. Olsson, and G. Wall 1976), and a summary of this paper was published in the same newspaper on the 6th of December, 1975 (B. Eriksson, K.-E. Eriksson, K. Olsson, and G. Wall 1975). In his article, Alfvén compared the energy accounting, irrespect of different energy values, with a cashier counting his cash only by the number of coins or notes, and neglecting their value. This comparison has a striking similarity with what is happening in the energy description of a space heating system. Here one describes the exchange of an "one hundred kronor note" (SEK 100), i.e., electricity, into a "five kronor coin" (SEK 5), i.e., heat at indoor temperature, as if their value did not matter. Unfortunately, the pedagogical content of these newspaper articles is still as much relevant as it was eleven years ago.
Few areas of science and technology have been so closely related to public debate as energy research. Since the 1973 oil price rise the debate on new energy sources, energy conservation, and energy-environment relations has continued. Recent events, such as the nuclear power plant accident in Chernobyl and the increased effects of acid rain on forests, have put new fuels into the debate. No simple solutions exist, mainly because the situation is strongly related to the goals of the modern society. Large use of energy and material resources based on mineral exploitation, with today's technology, cannot continue together with a healthy environment. This situation can only be changed by changing the aims and the direction of today's society. Fundamental scientific concepts and methods to describe reality must play an important part in this process of change. Some ideas are presented in this thesis.
Chapter 2 gives a short description of physical resource theory, a new scientific field which has connections to several other research fields, such as physics and economics. This description also gives a background to the research area covered in this thesis.
Chapter 3 describes the milestones in the development of the exergy concept, which is further treated in the first paper of the thesis, Exergy - a useful concept within resource accounting, described in Chapter 4. Paper I also includes a first attempt to apply exergy to the energy and material resource conversion system of a society (Sweden 1975). This application has been developed further (Wall 1978 a, 1978 b, and 1983 b) in Paper II, Exergy conversion in the Swedish society, which is also described in Chapter 4. In order to give a historical perspective of today's resource use in Sweden I have also included a description of the situation in Sweden in 1920 (Wall 1982).
Chapter 5 (Papers III and IV) presents two rather straightforward applications of the exergy concept to technical systems. Paper III, Exergy flows in industrial processes, is one example of how industrial processes could be described in terms of exergy and what the benefit would be of such a study. The paper includes all necessary tools, even lists of computer programs. Paper IV, Thermoeconomic optimization of a heat pump process, goes a bit further in its analysis by including economic objectives. This is an important improvement, since it immediately answers the question: Is a particular technical improvement also economical? The method is basically simple, but, applications to real systems imply large and sometimes even impassable obstacles. Numerical treatment may partly solve this problem, as is shown in the paper.
Also, a bibliography is added as a step towards the development of a data base in this field.
In nature there are physical systems which process energy and various materials and which thereby build up and maintain ordered structures. Examples of such systems are living organisms and ecosystems with living organisms in interplay with one another and with the non-living environment. Also in human settlements and societies a similar conversion of energy and materials is taking place.
Structural organization in matter is most appropriately described in information- theoretical terms. Furthermore, the systems often have control systems which process information. This information is physically tied to relatively small amounts of energy and matter. An important example is the genetic information in living organisms.
Energy, materials, and information will in this context be denoted by the common term physical resources.
Physical resource theory is the science dealing with physical resources and their conversion in various systems. The systems can be societal (e.g., technical, such as energy conversion systems or an industrial process), geophysical (e.g., the atmosphere or a mineral deposit), or ecological (e.g., an ecosystem or an organism). Special attention should be given to the conversion of physical resources in societal systems. This has to be studied with reference to human needs, availability of resources and the possibilities of incorporating these conversions in the natural system. Another important task for physical resource theory is to develop methods to optimize resource conversion processes. The systems are described and analyzed by means of the methods of mathematics and the natural sciences.
Thus, physical resource theory deals with questions that arise within science itself and with problems in connection to the resource handling of the society.
Never before in the history of science have macrocosmos and microcosmos been so intimately tied together. There is as yet no complete theory of elementary particles, and structures of dimensions from 10-19m down to 10-35m still remain to be
explored. (The smallest dimensions may be inaccessible to observation due to difficulties in achieving the enormous energies needed to penetrate into this region.) Even if new and surprising phenomena are observed they will not separate the physics of microcosmos from that of macrocosmos but rather tie them more strongly together in an effort to understand the beginning of the universe.
A frontier of physics which is becoming increasingly important, besides the microcosmos and the macrocosmos, is the physics of the complex. This could involve branches of physics that have become sciences of their own, e.g., atomic and molecular physics, solid state physics, nuclear physics, astrophysics. However the frontier which may change scientific thinking most is rather the physics of self-organizing systems.
Prigogine (1980) also uses the term "dissipative structures" to denote self-organizing systems, thereby indicating that such systems dissipate energy to build up new structure. Haken (1980 ed., 1983, and 1984) has shown how many degrees of freedom are tied to ("slaved by") a few degrees of freedom, described by a few "order parameters". Structure may also be described in information theoretical terms: dissipative structures create new information while dissipating energy. Dissipation of energy can also be described as consumption of exergy, i.e., of energy weighed according to quality. Thus dissipative structures use exergy as an "input" to produce structure/information. Some of the exergy is tied to the structure, some is consumed in the process.
Since information increases, dissipative structures are inherently indeterministic, the information content of a system at one time is in general insufficient to predict the state of the system at later times. Small unpredictible fluctuations may have a decisive influence on the whole system. The system builds "order through fluctuations" (Prigogine 1980).
Exergy can thus be destroyed ("consumed") but not created - except at a cosmic scale due to changing local equilibrium conditions in a changing universe (Eriksson, Islam, and Skagerstam 1982). This is a consequence of the second law of thermodynamics. All this means that exergy is the resource, consumed by dissipative structures that use exergy to produce structure/information, as well as by decaying structures or non-structured systems which go towards equilibrium or towards a stationary state. "Structure" is here a spatial or temporal order describable in information-theoretical terms (Eriksson and Lindgren 1986).
There is a good reason why exergy should be used as a resource measure rather than negentropy, as has often been suggested. The reason is that mechanical work W is pure exergy E, E = W, whereas the negentropy S contained in work depends on the ambient temperature To, S = W/To. It is also convenient to use a concept that is directly related to mechanical work.
According to the current theory of the origin of the universe, at 0.01 second after the start (the Big Bang) thermal equilibrium prevailed everywhere. The exergy was zero, since in the absence of any gradients no work could have been extracted. Now the situation is different. In an overall cold and thin gas very hot and dense bodies like our sun move around. There is now exergy. The water cycle of the earth uses the sun as a boiler and the space as a cooler to extract work. There are many levels on which resources are converted and exergy is consumed: galaxies, quasars, stars (including super novas).
When did this exergy arise, and how? The answer is that the cosmic exergy is mainly nuclear and that its creation started during the first three minutes described by Weinberg (1977) and Eriksson, Islam, and Skagerstam (1982).
Our planet is itself a resource converting system. Several subsystems can be identified and they are worth special studies: the interior of the earth and the crust of the earth, its surface and the atmosphere. The last two systems are of particular interest since they contain the life-supporting system, the biosphere, which includes the water cycle and other similar cycles and in which the human society is embedded. Next come ecosystems and their populations of various species. Finally we come down to the metabolic cycles of the living cell.
At all levels there is creation of information. For instance, the solar radiation impinging on the earth is a photon gas very near equilibrium and containing very little information in the sense that it is very simple to describe (Chaitin 1979). (We disregard here the information on the sun's surface which is irrelevant for this context.) But the solar radiation is much hotter than the earth's surface, it has relative to the earth a lot of exergy. And out of this exergy (information capacity) comes new information in a continuous coding process - a truly creative process. Most of the systems that use the terrestrial exergy have long been studied within the natural sciences. Such studies include the turn-over and transfer of energy and matter (materials) and accumulation of information in the systems under study. However, a consistent accounting, exergetics, seems to be lacking in most of the relevant study areas.
Human societies are - among many things - also physical systems, converting resources. To view them as such is therefore a valid point of view, see Section 2.2.2.
So far, we have been discussing resource-converting systems. In order to understand those it is necessary to have a clear picture of the relations between physical quantities within a given system. This is necessary before systems studies can be made successfully. We shall now focus on the exergy concept's relation to various forms of resources and to other physical quantities.
Even the simplest forms of energy, mechanical energy and heat, have not been studied in detail until recently with regard to this relationship. However other energy forms are in greater need of study with respect to their convertibility into work. Such studies are included in what we call exergetics (Eriksson 1982 a). The following areas are then of importance:
Such work also serves the purpose of giving a more firm physical foundation to the description of societal resource conversions, see Section 2.2.2, where uncertainties and ambiguities clearly have political implications.
The concepts and relations of statistical mechanics and information theory are so general that they may be applied to a large class of systems irrespective of the details of those systems. This opens the possibility of combining statistical mechanics/ information theory with another general theoretical framework, system theory, into a general description of resource-converting systems, e.g., ecological systems (Eriksson and Kåberger 1984). The above-mentioned work on self-organizing systems, called synergetics by Haken, is of great importance here. Relevant concepts and models have also been developed within ecology and economics. It would be very valuable if one could develop a simple diagrammatic description of resource-converting systems.
The following questions naturally arise within this context (system exergetics):
The above discussion may give the impression that physical resource theory covers almost everything. In a way this is also true - as it is true that physics covers almost everything. But then one must bear in mind that this can only be so because the aspect is very limited. Physics can deal with such a wide realm of phenomena only by choosing one or a few very limited general aspects. The same is true for physical resource theory. The arguments given here support the view that those limited aspects are relevant to the natural sciences. We shall now argue that they are also relevant for the description and understanding of processes in society.
As stated already, a society may - among many other things - be viewed as a physical system. As such it is embedded in and draws its resources from one or several natural systems.
During the 1970's it became increasingly clear that what is commonly called "energy" is a crucial resource for a society. Whereas matter is conserved (disregarding radioactive decays and nuclear reactions, the chemical elements are conserved) and, in principle, possible to use over and over again, energy, although it is also conserved, can be used only up to a point where it has lost its quality. Also degradation or spreading out of matter (materials) can easily be described in exergy terms.
The intimate connection between "energy" and materials is obvious in a variety of cases:
If resources are so closely tied together it seems reasonable to try to study them within a unified theoretical framework.
In the discussion above, we have tried to outline such a framework and describe its place among other natural sciences. We have also discussed questions that arise, and possible applications of the answers to those questions or the methods developed.
What can be of interest in a societal context are those applications that refer to natural systems and processes which are of great importance to the society or those which concern various components of the society's resource system. Methods developed and tested within a natural science context may also be useful when applied to the society.
Exergy has to be spent in any process which takes place at a non-zero rate. The exergy exspenditure can - to a large extent - be steered by will. Exergy is thus a physical measure of the action potential of the one (or those) who command it. Clearly it is by no means a complete measure. Availability of efficient technical equipment as well as relevant know-how and rational organization are of great importance, and the action potential may, accordingly, involve the option to invest in equipment, know-how, or organization.
This comment, although very sketchy and incomplete, indicates that exergy is a concept of high relevance to economics. Since exergy is a measure of contrast within a system or between a system and a given environment it comes very close to a measure of value. (With this measure, environmental disturbances like pollution have an immediate exergy cost, since the contrast is reduced when there is partial mixing (between different systems).)
It is an important task for physical resource theory to develop useful physical methods for describing stocks and flows of resources within an economy, including the flows in and out of it and the transformations within it.
Although basically physical, such descriptions should be designed to be as consistent as possible with economic terminology. In the literature, various degrees of crudeness or fineness have been applied in the characterization of resources. One approach to this is the method known as energy analysis (Johansson and Lönnroth 1975, Thomas 1977, Gilliland 1978, and Chapman and Roberts 1983). In our case it would rather be an exergy analysis (B. Eriksson, K.-E. Eriksson, Olsson, and Wall 1976 and Wall 1977), which would use the results of the system exergetics (Grubbström 1980 and Eriksson 1982 b ). A general description method could probably be developed, which can be used to achieve an arbitrary fineness in the resource characterization.
Since the society draws its resources from and returns residuals to some natural system, some descriptions of the natural system or parts of it should be included.
During the 1970's, economists, inspired by the big world models, discussed intensively how to describe and optimize resource use over time, and many models were designed for the handling of this problem (Meadows and Meadows 1972 and 1977). As pointed out by, e.g., Ayres (1978), the physical constraints on the efficiency have not always been properly taken into account in this modelling.
This sort of work is nevertheless important as a basis for the discussion on resource planning. One problem, besides physical constraints, that needs further elaboration is the interplay between non-renewable and renewable resources. There are also many questions that could be given new answers in this context and which should therefore be open to discussion:
The usefulness - or the utility - of certain goods is not directly a function of the goods themselves but rather of the services that they can provide. For a given set of services or functions, one can then analyze its costs in terms of exergy and materials or labour. Various ways to provide the same set of services may then be compared, and their technical and organizational efficiency may thus be evaluated. For instance, the same indoor climate may be achieved in several ways at widely different resource costs.
Due to structural, administrative, or legislative restrictions, or even lack of imagination, the optimal way may often not be able to compete on a market. One aim in a study of organizational efficiency is to provide information which cannot be transferred by the market. We may also look for and take into account constraints from ecological or ethical limits. An important problem is to minimize resource costs for a given set of services under such constraints.
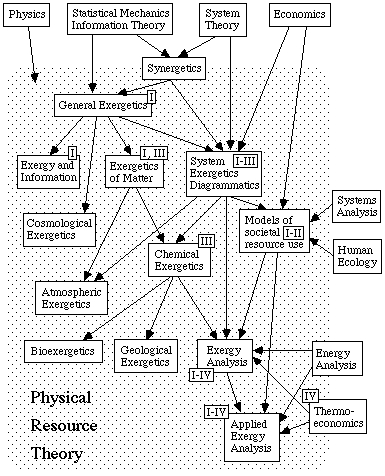
Physical resource theory, originating from physics, has to process knowledge from many fields of science. The main sources of theory are statistical mechanics, system theory, including synergetics (the theory of self-organizing systems, Haken 1980 ed., 1983, and 1984), and economics (see Fig. 2.1). In order to investigate different systems one also has to collect information from other branches of physics and other natural sciences.
With human ecology (Eriksson, Islam, and Tengström 1981 and Tengström 1985) the relations are a bit different. Human ecology is the study of the interactions between man and his/her total environment. The relations between a society and its natural environment is of fundamental importance. So are the relations between societies. A study of this kind must be interdisciplinary in a broad sense. A close cooperation and integration is necessary between natural sciences on one side, and social sciences and humanities on the other. The two sciences have similar aims, and the conceptual problems are partly the same in both sciences. Human ecology is the one with the broader scope, and physical resource theory is the one which is more method-oriented. Physical resource theory could serve as an auxiliary science to human ecology, and human ecology could help setting values to be applied in physical resource theory. The integration of knowledge and the development of concepts should be done in close collaboration between the two.
Although there is no arrow in the chart back into the economic box, one may hope that developments in physical resource theory will lead to results which benefit economics. Work by Grubbström (1980) and Månsson (1985 b) indicate this.
Systems analysis has a very wide definition (Patten 1971, Pantell 1976, Bell et al. 1977, and Bennet and Chorley 1978) and is more related to engineering and social sciences. However, several of the methods and applications used in physical resource theory also play a role in systems analysis (Wall 1981, 1983 a, and 1985).
Thermoeconomics, which is described in Chapter 5, is strongly related to exergy analysis and applied exergy analysis (El-Sayed and Tribus 1983, Wall 1985 and 1986).
The boxes in Fig. 2.1 are marked with numbers indicating where the papers in this thesis belong.

In 1824, the French engineer Sadi Carnot published a relation between heat and work which later resulted in the formulation of the second law of thermodynamics. J. Willard Gibbs was the first to express the general relation for work as early as 1873.
"We will first observe that an expression of the form
denotes the work obtainable by the formation (by a reversible process) of a body of which e, s, v, m1, m2, É mn are the energy, entropy, volume, and the quantities of the components, within a medium having the pressure P, the temperature T, and the potentials M1, M2, É Mn. (The medium is supposed so large that its properties are not sensibly altered in any part by the formation of the body.)"
Not until 1953 did Z. Rant suggest the name exergy.
"Aus diesen Forderungen geht hervor, daß ãie" die zweckmäßigste Nachsilbe sein wird. Da es sich bei dem untersuchten Begriff um eine Arbeit handelt, muß als Stammsilbe (als genus proximum) das griechische Wort erg (on) hierfür erscheinen. Nun ist noch die richtige Vorsilbe zu wählen, die die spezifische Eigenart, die differentia specifica, hervorhebt. Hierfür gilt die Forderung, daß der neue Begriff die Arbeit bezeichnen soll, die aus einem System herausgeholt werden kann. ãAus" heißt auf Griechisch ãek" vor Konsonanten bzw. ãex" vor Vokalen.
Damit lautet der neue Begriff Exergie: er erfüllt praktisch alle aufgestellten Forderungen, und der Buchstabe x unterscheidet ihn klar vom verwandten Begriff der Energie, so daß trotz der Analogie in der Wortbildung jede Verwechslung ausgeschlossen bleibt. Der Ausdruck kann in jede germanische, romanische oder slawische Sprache eingeführt werden, er lautet z. B. auf deutsch Exergie, auf englisch exergy, auf französisch exergie, auf spanisch exergia, auf italienisch essergia und auf slawisch eksergija."
A general definition was given by H. D. Baehr, 1965:
"Die Exergie ist der unbeseschränkt, d. h. in jede andere Energieform umwandelbare Teil der Energie."
These three works constitute an adequate definition of the exergy concept, thus establishing a foundation. However, some later publications deserve to be mentioned, such as a special issue of Energy (Penner 1980) and some textbooks: Gaggioli (1980), Ahern (1980), Edgerton (1982), Gaggioli (1983), and Moran (1982). Richard Gaggioli makes the following statement (1980):
"The concept of exergy is crucial not only to efficiency studies but also to cost accounting and economic analyses. Costs should reflect value, since the value is not in energy but in exergy, assignment of cost to energy leads to misappropriations, which are common and often gross. Using exergy content as a basis for cost accounting is important to management for pricing products and for their evaluation of profits. It is also useful to engineering for operating and design decisions, including design optimization.
Thus, exergy is the only rational basis for evaluating: fuels and resources, process, device, and system efficiencies, dissipations and their costs, and the value and cost of systems outputs."
In science and technology one has used, for a long time, thermodynamic potentials similar to exergy but more limited in scope. Gibb's free energy, Helmholz' free energy, and enthalpy are all special cases of exergy (Evans 1969, Wall 1977, Andersson, Fredriksson, Ljung, Söderström, and Wall 1981).
We may express the energy and exergy concepts in the following simple terms: (1) Energy is motion or ability to produce motion and (2) Exergy is work or ability to produce work. The laws of thermodynamics may be formulated accordingly: (1) Energy is always conserved in a process (First law, the law of energy conservation) and (2) Exergy is always conserved in a reversible process, but is always consumed in an irreversible process (Second law, the law of exergy).
The historical development of the concept of exergy should be documented. I see this as an important task especially after preparing the bibliography which indicates that a lot of scientific work is never accepted in society or applied to real processes. Thus, I strongly recommend this as a topic for a study in the history of science.
It is important to investigate the basic physical premises for human societies on earth. Questions concerning the resource situation and the state of the environment play an increasing role in the society. It is therefore important to have an adequate description of the resource conversion processes in a society and a general method to make such descriptions.
In Paper I, Exergy - a Useful Concept within Resource Accounting, the exergy concept is discussed in this respect. It deals with the theory of exergy applied to matter, its relation to other thermodynamic potentials and to information theory, the calculation of exergy of an ideal monatomic gas and the comparison of information transfer efficiency between technical and biological systems. In Paper II, Exergy Conversion in the Swedish Society, this is described more in detail. Paper I describes the situation in 1975 and Paper II describes the situation in 1980. The difference is mainly the increased use of nuclear power and the decreased use of fuel oil for space heating. This method of description is an improvement over conventional energy flow diagrams in two ways. First, since material flows are included, it gives a more complete picture of the resource conversion in a society. Secondly, since energy quality is taken into account, the method immediately reveals in which conversions it is physically possible to improve the efficiency.
Since Paper I is an early paper, a few minor modifications are motivated such as a unit shift to J instead of Wh, and the following comments:
(1) Table 2.1. on p. 12: Note that for some substances the exergy content may even exceed the chemical energy content, due to definitions of system boundaries and final states.
(2) Table 2.2. on p. 13: Note that matter in an ordered form also may include biological organisms, e.g. a living plant.
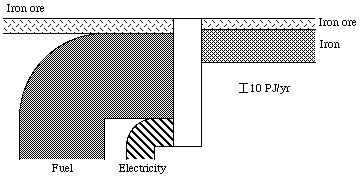
(3) Figure 3.7 on p. 27: By using the reference states of Paper II for iron, Fe and O are represented as Fe2O3 (hematite) in solid form at a mole fraction of 2.7x10-4 and O2 in gaseous form at a partial pressure of 20.40 kPa in the standard environment, we get a different description of the Swedish iron production in 1975. The Swedish production of iron ore in 1975 was approximately 36 Mtons. If we assume all this ore to be magnetite iron ore, as most of the Swedish iron ore is, then the ore represents a total exergy quantity of 18 PJ. The production of iron was roughly 6 Mtons, representing an approximate quantity of 41 PJ. To produce this iron, about 10 Mtons of ore was needed, corresponding to 5.1 PJ together with 36 PJ of electrical exergy and 110 PJ of coal, coke and other fuels. The exergy efficiency in the iron production process then becomes about 27%. Figure 4.1. illustrates the situation.
(4) A diagram of exergy flows can be constructed for the total conversion of energy and material resources which takes place in the Swedish society during one year. This will look like the diagram in Fig. 4.2. This description differs somewhat from that of Paper I (Fig. 3.9, p. 31), but corresponds better with later studies. The inflows of energy and materials origin from the resource base, which is represented as a box in the left part of Fig. 4.2. The outflowing "products" are difficult to define in a uniform way. The individual, however, plays an important role as the final user, by directly or indirectly demanding the "products". This is indicated as a box in the right part in Fig. 4.2.
The total conversion of resources is about 2500 PJ/yr or 300 GJ/yr per person and the net yield is about 450 PJ/yr or 55 GJ/yr per person. At the top of the chart is the inflow of sunlight (about 20 PJ/yr) which is converted to indoor heat, about 1 PJ/yr. The next conversion in the diagram concerns the forest industry. The stock of forest, a fund, is harvested each year and the timber is used either as timber raw material or converted into paper pulp and paper. At the conversion of timber into pulp, large amounts of heat is used to boil wood chips in the processing of paper pulp. This heat is produced by burning effluence (liquors) and fuel oil.
In 1975, the estimated net felling in Swedish forests corresponded to about 430 PJ. The greater proportion of this quantity (200 PJ) went to the sawn timber industry which, in 1975, produced 94 PJ of timber, 61 PJ of by-products for the pulp industry and 14 PJ of by-products such as firewood. The pulp mills were supplied with 200 PJ of timber, including the above-mentioned 61 PJ of which reappeared as pulp and 86 PJ as paper in the end products. The main losses incurred when sawing trees into timber are in the form of waste and sawdust. In 1975, these losses amounted to 31 PJ. Paper pulp manufacture is extremely wasteful as far as exergy is concerned, due mainly to the amount of heat required for digesting wood chips. This heating requirement accounted for about 130 PJ of the wood harvested. Together with the 105 PJ of fuel supplied, this combustion process contributed less than 60 PJ of heat and about 10 PJ of electricity, which was used in the forest industry. The pulp and paper industries accounted for a further 53 PJ of electricity.
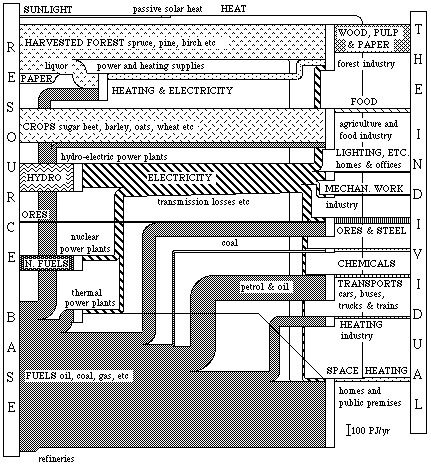
The exergy content of the end product, which consisted of wood, pulp and paper, was 250 PJ. The efficiency of the conversion for the whole of the forest industry was thus about 42%.
The next conversion processes shown in the chart are agriculture and the food industry. The total exergy content of the products of cultivation was 105 PJ. In addition, fodders and waste accounted for an estimated amount of the order of 200 PJ. Thus, the total annual crop exergy was about 300 PJ. In addition to crops, agriculture and the food industry accounted for the conversion of a further 50 PJ of fuels and 13 PJ of electricity for machine power and heating applications. In this sector, the end product is food and a daily intake of 12 MJ per person is equivalent to an annual conversion of 36 PJ for the country as a whole. This means that the food which is thrown away is not included in the food flow, but is represented as a loss in food processing. Approximately as much as 25 - 30% of the food that leaves the shops is thrown away. Large parts of the losses in agriculture are also inevitable since some parts of the crops are not edible to humans, such as straw. Regrettably the use of straw in animal production has decreased considerably the last few years. This depends to a large part on the shift in the consumption of animal products from milk and butter to pork and poultry. Therefore, the efficiency of the conversion within food production becomes quite low, only about 9%. Also, the exergy content of the agricultural produce is lower than the exergy content of the inputs such as fertilizers, machine wear, and fuels. About a third of the inputs of fuel and electricity in food production is used in the food processing industry.
Hydro-electric power is the next conversion process shown in the chart. In 1975, the electricity was also used for lighting, domestic power supplies etc (82 PJ). The engineering industry used a great deal of electric power to drive machines, i.e., to perform mechanical work (about 26 PJ). The use in the forest industry and in food production was mentioned earlier. The remainder was used in the mining, iron and steel industries (36 PJ), the chemical industry (21 PJ) in transport applications (7 PJ) and for electric heating (22 PJ).
In 1975, the production of electricity from hydro-electric power sources amounted to 208 PJ. If we assume the losses in converting the potential energy of the water in the reservoir into electricity from the power station to be 15%, this corresponds to a total exergy requirement of 244 PJ.
Nuclear fuel (U-235) and fuels such as oil were also used for generating electricity, the conversion being carried out in condensing power stations and combined power and district heating plants. Apart from generating electricity, the latter type of plant supplies district heating by a so-called back-pressure process. The chart illustrates how this flow of district heating (6 PJ/yr) is distributed as a heat outflow to homes and public premises. The chart also shows that only one-third of the nuclear fuel is converted to electricity, the remainder being lost in the conversion process itself. The losses in nuclear, condensing and district heating power plants are of the order of 60%.
In 1975, the production of electricity from nuclear and hydrocarbon fuels amounted to 43 and 40 PJ respectively. To this must be added the power station house loads including losses in power transformers and pumping losses in pumping stations. Thus, total production of electrical energy in 1975 amounted to 295 PJ , of which a net 4 PJ was imported. Of this production, 260 PJ was actually consumed, the remainder being represented by losses in transmission and distribution to the consumer.
In Sweden, iron ore accounts for almost all of the ores converted. In Fig. 4.1 the Swedish iron ore conversion process was presented. We see this conversion process in its context in the diagram in Fig. 4.2.
The most common fuels used in Sweden are crude oil, oil products, coal and coke. In 1975, imports of these products amounted to a total of 1 323 PJ.
Fuels are used as feedstocks in the chemical industry. In 1975, 18 PJ of oil and 21 PJ of electricity were converted into about 30 PJ of rubber, plastics etc. Thus, the chemical industry supplies an example of how a traditional energy resource such as oil is used as a feedstock and how the product itself can be used as an energy source at the end of its life. Naturally, this also applies to many other "used" materials such as wood and paper.
As we can see from the chart, transportation accounts for a major proportion of the fuel inflow (220 PJ/yr). Petrol and oil are converted to motive power in cars, buses and trucks. About 10% of the exergy content of the fuel is used to propel a motor vehicle (about 1 ton of steel) forwards. The remainder is either lost or is expended in wearing out the exhaust system, engine and tyres of the vehicle.
As regards the remainder of the originally listed areas of consumption, 36 PJ are supplied to the oil refineries, about 583 PJ for direct conversion to heating in homes and other premises, 115 PJ for the production of electricity and heat in thermal and combined power and district heating plants, and 86 PJ for the production of heat etc in industry.
The largest conversion process - that of fuels, solar heat, district heating and electricity to heat - is illustrated at the bottom of the chart. As we can see, this conversion process, which is divided between industry, homes and public premises, entails appreciable losses. In a conventional oil-fired boiler, less than 5% of the fuel exergy content goes into the heat produced. Half of the imported oil is used for heat production.
The exergy content of heat is determined by its temperature as defined by the formula:
|
|
(8) |
If we now wish to use this heat for heating homes, we must also allow for the fact that the environmental temperature is subject to seasonal variations. Thus, Swedish residential heating requires a net exergy flow of 0.05 times the quantity of heat (energy) supplied. This means that the exergy contents of the various heating flows were: solar heat 1 PJ/yr , district heating 2 PJ/yr , electric heating 1 PJ/yr and heating produced by fuel burning 19 PJ/yr. The latter figure also includes other direct energy losses such as flue gas losses (amounting to about 35%).
Of the total national inflow of energy and material resources (about 2 500 PJ/yr) in Sweden in 1975, only 18% or just over 450 PJ/yr was used. The loss which this represents could be reduced appreciably by active conservation in the society. Looking at the utilization of commercial energy resources alone, the efficiency is somewhat lower (about 12%).
(5) It is difficult to apply the concept of information to biological systems. In a sense it is meaningless (biologically) to speak about the information content of a chromosome without regarding at least the system surrounding the chromosome, i.e., the biological organism. However, all biological organisms are related within the biospere. The ecological evolution is a result of interactions within this system. Thus, to extract a part of this system and evaluate it in physical terms has, of course, only physical meaning. The protein biosynthesis presented in the information rate versus power diagram in Fig. 4.1, occurs in an environment difficult to define, and the information is transferred in packages of information. A package of information can, naturally, be transfered (transported) between systems with hardly any exergy. An information content may thus be transfered well below the line indicating the ambient temperature in Fig. 4.1. In technical systems this may be done e.g. by cooling the components. The visualization must, however, occur well above this line.
(6) In relation to the discussion on human utilization of exergy and information (Eq. (12), p. 37), the following simple calculation might be added. The total inflow of information since the creation of the earth accounts for about 4x1054 bits, an incredible amount of information. After a few billion years life began on earth and just recently homo sapiens entered. The immense information capacity inflow plays a crucial role for the existence of these phenomena on earth.
In order to give a historical perspective of today's resource use in Sweden, given in Paper I and II, I also add the descriptions of the exergy conversion in the Swedish society in 1920 (Wall 1982).
This description is based on a study from the Centre for Interdisciplinary Studies of Human Conditions at the University of Göteborg (Egnéus et al.1978).
For the Swedish population the increased use of resources meant an increased material standard at this time. In 1920 there was no longer any self-sufficiency on a household level. About half of the population depended on a monetary income and had to pay for the greater part of their basic material needs, such as food, clothes and living quarters. The food that they bought was very satisfactory measured by the standards of the 1970's. The construction of new houses increased and older houses were improved. Better living conditions and better hygiene were to eliminate certain diseases later on, mainly tuberculosis. The improved standard of living removed the soot and ashes from the living quarters to the surrounding air, and human waste was removed from the privy in the backyard to the water courses. The people of the 1920's did not see the negative consequences of this.
The supply of energy and material resources was important for all these changes. Exergy was necessary to take care of the flows of resources, to convert them into useful commodities, and to transport these to the consumer.
In the towns, there was a shift from the use of Swedish resource flows to using imported deposit resources such as coal and coke. The car brought with it imports of fuel from abroad, mainly USA. However, firewood was still important locally. Both industry and households were thus well prepared for a reduction of imported fuels. Water power became more and more important both in industry and in households during the 1920's. Industry produced more commodities for ready consumption out of raw materials.
When resources were increasingly fetched from peripheral areas, and all values were translated into SEK, people's awareness of scarcity, of the need to limit the extraction from funds and of the finite nature of deposits, decreased.
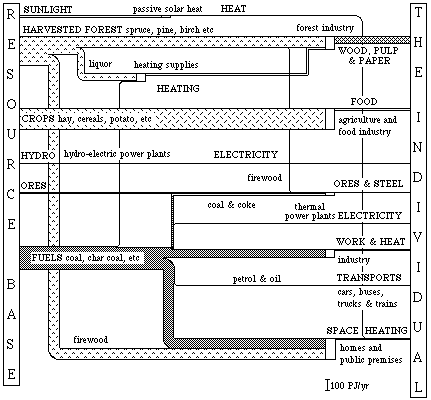
In the flow diagram below, the Swedish energy and material conversion in 1920, in terms of exergy, is illustrated. Some of the figures are only estimated. A more detailed description of the resource conversion follows below.
The first technological revolution came with the introduction of a new source of power, the steam engine, the second with electricity, which brought with it a new technique for the transfer of power as well as the combustion engine, and the third which occurred after World War II (WWII), with electronics and computerization.
Electricity: The first real breakthrough in the use of electricity in industry came with the development of the three-phase system during the 1890's. It was this system that was the important factor in the industrial revolution at the turn of the century. At first, electric lighting was introduced with the light bulb, and electricity was produced in thermal power plants. Electricity, however, could also be derived from hydro-electric power. The use of electricity for driving industrial machines became, with the three-phase system, an interesting alternative to the more direct use of water wheels and steam engines. However, the factories had to be rebuilt. The use of electricity in industry created a base for an expansion of hydro-electric power to the larger and more distant waterfalls. The use of electricity in industry, the possibilities of using large amounts of electricity to power electric furnaces as well as for electrolysis, put those countries that had plenty of large waterfalls, such as Norway and Sweden, in a more favourable position.
The electric furnace, the process of electrolysis, and the use of electricity to produce several of the most important semi-manufactures in chemical industry - ammonia, calcium carbide, chlorine, chlorate - were also part of the great industrial revolution. But electrical energy was expensive to produce, and electric furnaces, electrolysis and electricity in the chemical industry needed a lot of energy per product unit. Therefore, they only became interesting when power plants and the distribution network had been built, and the electric power plants had reached a surplus capacity.
The operation of railways and trams is yet another use for electrical energy. Apart from small industrial railways and short tramways, it was not until well into the 20th century that electric locomotives and trams became common. This is partly because there was not enough electrical energy at first to run larger railways, and partly because the addition of railways to a common distribution network caused disturbances in both electric and telephone networks.
Petroleum: The use of petroleum was another important factor in the great industrial revolution at the turn of the century. Small quantities of oil were already used for lighting (paraffin oil for lamps and paraffin stoves) and for greasing, but there were no uses for the lightest fractions, i.e., petrol, and the large-scale use of the heavier fractions as fuel had not yet started in the 1870's. Society was adapted to the use of solid fuels, and before petroleum was able to compete on a large scale a distribution system needed to be established and drilling techniques needed to be improved and to become cheaper. These investments were not made as long as there was still plenty of coal in the industrial centra of the world and fuel-wood in its peripheral parts.
In the 1920's, oil was still little used as compared with coal, but the breakthrough can still be said to have come during the decade. The cause of the breakthrough was the possibilities that oil had in new areas. One was the ability to use existing machines better. This was interesting, for example, in the case of steam turbines used to generate electricity. Turbines have advantages over, for example, the steam engine, in driving an electric generator, mainly because they can produce the higher speed needed by the generator directly. As electric power systems were expanding, the need to regulate the production of electricity to answer demands became greater. The cost of fuel for this production of "peak power" was not so important if one could get away with low investment costs. Another possibility was to attach oil furnaces onto steam boilers usually fueled with coal, which could then produce more steam at a higher pressure and temperature through the more efficient oil combustion.
The internal combustion engine: The use of petroleum was, however, influenced by the development of the combustion engine for boats and cars, more and earlier than the possibilities for increased effect in some machines. The decisive factor was that engines with internal combustion could be made more compact and lighter in relation to the driving force produced than steam engines or other engines with external combustion. The weight and the space required for the brought fuel made the combustion engines quite superior to electrical engines.
During WWI, diesel engines were introduced on larger ships. This and the use of steam engines run with oil created better performance and less work on board, but were not as revolutionizing as the introduction of combustion engines, e.g. compression- ignition engines, on small motorboats. Fishing boats at sea, by coasts, and on lakes and rivers were fitted with engines.
New materials: Even if the introduction of electricity and the combustion engine were those aspects of the industrial revolution that meant most to development, there were several other aspects.
New materials were put into use. The use of electrolysis for the production of aluminium made clay into a technically and socially useful natural resource. The electric furnaces made it possible to produce alloys for steel in large quantities.
The effect of the alloying materials (as well as that of coal) on the properties of the steel were thoroughly studied from the beginning of the 1890's. Alloys with greater tensile strength, flexibility, and hardness were looked for. Soon also the interest in rust resistance increased.
Manganese steel was used early for certain construction purposes that required a high tensile strength. Chromium steel, which was very tough, was used for tools, machines, and military equipment. Wolframite or wolframite/molybdenum steels, which were very hard even after heating and cooling in air, were used for cutting.
Several alloys were known and used earlier as well, but then they were very exclusive because the components were rare and expensive laboratory products until the electric furnace was invented.
Special alloys made a much more effective workshop technology possible, and a new generation of machines was developed: milling machines, the turret lathe and the grinding machine were added to the traditional lathe, the planing machine and the drilling machine. Also, a simple automization of the machines was done so that they no longer needed the complete observation and skills of one worker. The workshops could work with a much higher degree of precision. New measuring instruments were developed, for example the so-called gauge block. Through the increased precision it was possible to produce screws, nuts, shafts, cogwheels, belt pulleys and even more complicated machinery in specialized workshops, to be assembled in other factories. It became important to standardize machinery if the specialization was to work, and this was achieved, especially in Germany. Completely new machinery was also developed, such as ball bearings.
Table 4.1 shows the agricultural production for vegetable consumption. Here farming acreage, production in tons, and the amount of exergy are given. Losses in the form of plants consumed by insects, fungi and bacteria, or as straw, tops and subterraneous parts of plants, have not been estimated.
|
(1000 hectares) |
(1000 tons) |
(PJ) | |
|
Cereal for bread |
503 |
802 |
12 |
|
Potatoes and sugar beets |
90.5 |
1968 |
8 |
|
Other plants |
22 |
34 |
about 1 |
|
Total |
615.5 |
2804 |
21 |
Table 4.2 shows the agricultural products of plant matter which is converted by animals before consumption.
|
(1000 hectares) |
(1000 tons) |
(PJ) | |
|
Cereal for fodder |
1049 |
1661 |
26 |
|
Roots and potatoes for fodder |
174.5 |
3275 |
12 |
|
Hay |
1569 |
5965 |
91 |
|
Total |
2792.5 |
10901 |
129 |
The exergy content of the straw can be estimated at 38 PJ, since the production of straw was about as large as the harvest.
The figures of production given in the tables are gross figures. This means that not all is consumed by animals or humans, e.g. planting seed, losses in connection with flour-making and treatment of the plant matter, and storage losses.
|
(1000 tons) |
(PJ) | |
|
Cattle |
103 |
1.6 |
|
Swine |
141 |
2.3 |
|
Other animals |
11 |
0.2 |
|
Dairy products |
4077 |
12.6 |
|
Eggs |
0.2 | |
|
Total |
16.9 |
It is the primary exergy flow from plants to man that has been presented here. The inputs of exergy needed to set this exergy flow in motion, for example, the production of fertilizers, concentrated fodder, and tools, are absent. These inputs were, however, much smaller than today.
Other important contributions were pastures for cattle, sheep and goats in natural fields and in woods, as well as berries, mushrooms and game animals.
The estimates presented here are not complete, but they show the possibility of estimating the total exergy flows. The exergy flow from agriculture was more than 180 PJ/yr. The part that was consumed by domestic animals had an exergy content between six and eight times the part which was consumed directly by man.
Physical usefulness: The physical properties which form the basis of a description of the Swedish forest are: (1) the area of forested land, (2) the geographical distribution of the forest, (3) the amount of timber in different forest regions, and (4) the combination of tree species in different forest regions. These factors also influence the site quality class.
More than half of the total area of Sweden was forested in the 1920's. The proportionally largest area of forested land was in the southern part of Sweden. In absolute numbers, however, the largest area of forested land was in the north of Sweden. But large areas in the north are bare mountains, and if this is excluded, over 70% of the remainder is forested.
Certain parts of forest growth cannot be utilized by man. Part of the forest ecosystem is destroyed by fire. About 0.4 M m3 of forest was lost through fire each year. However, this loss was small compared to the total biomass of the forest, about 1700 Mm3.
Another factor which can be of greater importance is the effect of wind on the crops, i.e., storms. About 5 Mm3 of timber was felled during two bad storms in 1931 and 1933. The dead trees also become breeding ground for various harmful insects.
Parts of the forest waste is used by humans, mainly as fuel. In 1927, about 12 Mm3 of stump wood (stumps and tops) was left in the forests. About 3 Mm3 of branches were obtained, and about half of this was used as fuel.
The felled timber is used (1) as fuel (see Section 4.1.5), (2) for building and wood industry (saw mills), about 15.4 PJ/yr, and (3) for the pulp and paper industry, about 12.8 PJ/yr.
In the 1920's one spoke mainly of fuels and power in connection with energy, where fuels meant both sources of heat and chemical raw materials. Power included steam engines, water wheels, oil combustion engines, etc, which was used directly, as well as electric engines. The installed power capacity was mostly given in units of horse power, while the energy they converted was less interesting. No uniform concept of energy was used.
The different fuels used in the 1920's had, of course, different properties and could not easily replace each other. In statistics, they were instead reported as the heat of combustion that can be obtained from them. To simplify the figures the heat of combustion was often compared with that of "good English coal" and then expressed as so-called coal equivalents, see Table 4.4. This table also shows those solid fuels which were used at this time.
The heat content per ton obviously varies a great deal. The heat content of coal is 28 GJ/ton while that of coniferous wood is only 11 GJ/ton. The variations are especially great when the heat content is shown per unit of stacked cubic volume. We see that the fuels obtained from forestry are much more voluminous than those based on coal with respect to the heat content. In comparison with coal and firewood (trunk wood), the waste fuels are very voluminous. This is especially true of wood shavings. This means that as soon as there is a question of transporting waste fuels a longer distance, transport conditions are far more unfavourable than for firewood or coal fuels. Since the waste fuels in certain industries, mainly the wood industries, are as great as the total production by weight, the wood waste (including potential waste fuel) caused a storage problem.
|
per mass unit (GJ/ton) |
per volume (GJ/m3 stacked) |
coal volume (m3) |
|
|
Coal |
28 |
23 |
1.0 |
|
Coke |
27 |
12 |
1.9 |
|
Airdry birch wood |
15 |
7 |
3.4 |
|
Raw birch wood |
13 |
6 |
3.6 |
|
Airdry coniferous wood |
16 |
5 |
4.2 |
|
Raw coniferous wood |
11 |
5 |
4.2 |
|
Charcoal |
26 |
4 |
6.0 |
|
Peat |
14 |
3 |
7.0 |
|
Saw dust |
2 |
11 | |
|
Wood chips |
2 |
10 | |
|
Wood edgings |
3 |
7 | |
|
Wood shavings |
1 |
20 |
When used for heating, the fuels can replace each other in equal amounts of coal equivalents. But if a high combustion heat is needed, the fuel has to have a high enough energy density (i.e., combustion heat per kilogram). For a metallurgical process it can also be important to use a fuel that gives off low amounts of certain pollutants, such as sulphur.
Firewood: In the 1920's, firewood was still a very important source of energy. It contributed to about one third of the yearly fuel requirements and corresponded to about 4 million normal tons of coal equivalent, i.e., the same as the yearly import of coal. Table 4.5 gives a general summary of the use of firewood by the main users in 1913-1935. It shows how the use of fuel decreases, apart from during WWI.
|
Sector |
|
|
|
|
|
Industry |
0.8 |
1.4 |
1.0 |
0.9 |
|
Transports |
0.1 |
0.4 |
0 |
0 |
|
Domestic sector |
15.3 |
16.8 |
14.4 |
12.0 |
|
Total |
16.2 |
18.6 |
14.4 |
12.0 |
Peat: The maximum production of peat in Sweden was about 700 000 tons, in 1920. The increase in the peat production from 1916 to the peak year 1920 was about 500%. The decrease after the peak of 1920 was even faster than the increase, and in 1922 the production was again the same as in 1916. The increase and the following decrease of industrially produced peat for selling followed quite closely the changes in price of coal.
Peat replaced coal and coke for certain uses in certain geographical areas. The main characteristic of the peat production during the period of 1913-1930 is the extremely fast increase during the energy crisis and the following equally fast decrease after the crisis to a slowly vanishing production around 1930. Before and after the energy crisis, approximately half of the peat production was used in industry and the other half for domestic purposes and heating of premises.
Coal: Sweden had her own flow sources of energy, i.e., forests and water, and, in relation to the population, these were vast. In spite of this the country imported stored energy resources from deposits. The most important commodity imported was coal, the most important commodity exported was paper pulp. Before WWI, 99% of all coal imported to Sweden came from Great Britain, which also received most of our exported paper pulp. During the war Sweden also imported a great deal of coal from Germany.
During the years 1921-1925, Great Britain almost monopolized the Swedish import of coal. This had been the case before the world war as well. 1920 is the only year when a large part of the import came from USA. 1920 was the year of the great coal strike in Great Britain. Sweden had to get coal from other countries, mainly Germany and Poland, and after 1926 Great Britain no longer had monopoly of the export of coal to Sweden, but from this time besides Britain the imports mainly came from Poland and Germany. The situation was the same when it came to coke.
The industry consumed about 50% of the coal used in the 1920's. Coal and coke were mainly used by gasworks, the paper and paper pulp industry, and the manufacture of non-metallic mineral products except products of petroleum and coal. Industry used coal and coke partly for power generation, partly for heating. Another great consumer was the railways. They consumed about 20% of the total consumption. The rest was shared approximately evenly by shipping, gasworks, public works, institutions and households.
The import of petroleum increased during the 1920's from 50 million kilograms (1920) to 160 million kilograms (1930). During the same period, USA's part of this decreased from 91% to 36% and the price from 0.31 SEK/kilogram to 0.06 SEK/kilogram.
The industrial consumption of oil as fuel did not at all increase as fast as the import. The industrial consumption was at most 20-25% of the import. The remaining consumption is difficult to trace.
The state did not take any initiative whatever to encourage or discourage oil imports. Oil was allowed to flow freely into the country. This was not the case with petrol.
The consumption of petrol in 1920 was 43 million kilograms of which 80% came from USA, and in 1930 410 million kilograms of which 43% came from USA and 40% from Great Britain. The price was 0.72 and 0.11 SEK/kilogram respectively.
The import of petrol increased throughout the 1920's. From 1920 to 1930, the import of petrol increased by a factor of eight. The main consumers (about 90%) of petrol were, of course, the cars, which increased rapidly in number during the 1920's.
The liquid fuel which has been used longest in Sweden is paraffin. During the 1920's the imported paraffin competed with electricity that was produced within the country.
The import of paraffin in 1920 was 62 million kilograms of which 91% came from USA, and in 1930 79 million of which 35% came from the USA and 45% from Great Britain. The price changed from 0.47 SEK/kilogram to 0.12 SEK/kilogram.
During the 1920's the Swedish import of liquid fuels increased rapidly. Sweden thus became more and more dependent on the import of liquid fuels, and the increasing portion of our fund resources such as paper pulp in exports was needed to pay for the import of fossil fuels and vehicles. It was only in the case of petrol that the state tried to impose restrictions to decrease the import and, to a certain degree, the negative effects on the trade balance. In 1924, tax and import duty on petrol were introduced, the first energy tax in Sweden.
The use of electrical energy was well established and increased greatly during the 1920's. The industrial use increased from 2.1 PJ/yr on 1920 to 3.6 PJ/yr in 1930. The increase within the domestic sector was particularly great and rose from 0.2 PJ/yr in 1920 by a factor of three to 0.6 PJ/yr in 1930.
During the period 1915-1935, the use of electrical energy within the domestic sector increased, on average, by 10% per year. This rapid increase is probably connected with the shortage of fuel during and after WWI.
The main part of the electrical energy in the domestic sector was used for lighting in the towns. But it was during the 1920's that the absorption refrigerator was invented by von Platen and Munthers. The number of refrigerators increased from 500 in 1920 to 4000 in 1930.
The very rapid increase in the use of electrical energy was due not only to the decrease in price relative to fuels, but also to the fact that the state actively encouraged the expansion of electric power plants and power lines - especially in rural areas. However, electrical energy was still mostly too expensive to be used for other purposes than for lighting. The power plants and those industries which produced electric machines made strong propaganda for an increased use of electrical energy.
In all the diagrams of societies exergy resource use the inflowing resources come in an order with natural flows at the top, followed by flows from funds and flows from deposits. The inflow of solar heat is thus a direct exergy flow from the sun. Then follow the inflows of forest crops, agricultural crops and hydro-electrical power. The remaining inflows of ores, nuclear fuel and other fuels come from dead stocks, deposits, on earth.
In Table 4.6, the figures for Sweden in 1920, 1975, and 1980 are given. We can see that, besides an increased resource use, the resources today origin from mostly deposits instead of funds as in 1920.
|
capita GJ/yr capita |
funds % |
deposits % |
capita GJ/yr capita |
capita % | |
|
Sweden 1920 |
120 |
69 |
28 |
30 |
25 |
|
Sweden 1975 |
300 |
40 |
59 |
55 |
18 |
|
Sweden 1980 |
305 |
40 |
59 |
60 |
20 |
Paper III, Exergy Flows in Industrial Processes, gives a detailed description of the exergy flows in a pulp and paper industry and in a steel plant, two kinds of industries with heavy flows of matter.
The ability to find new solutions is often limited by the existing technology. Current technology is often overestimated in relation to past technology and to future alternatives. Thus, today's most sophisticated computers are mere "steam engines" when compared to the simplest biological cell or to future computers as is also illustrated by the rapid developments in the computer field. This paper emphasizes therefore the importance of defining the problem in scientific terms, that is, unhindered by the limitations of current technology. Such a description must, of course, be based on clearly defined scientific concepts. If not, we might be evaluating a false picture of the problem that may become an obstacle to important technological advances.
This paper presents in more detail the energy and exergy flows of two typical Swedish industries, a pulp and paper mill and a steel plant. These are also described in relation to Sweden's space heating system. The pulp and paper industry which I have studied produces unbleached kraft liner. After the wood is cleaned, debarked and chipped, the wood chips are processed in a continuous digester to a sulphate pulp. The separated bark is combusted together with fuel oil to produce steam and electricity, both of which are used in the process. The chemicals and the stripping (or draw-off) liquor leave the digester after processing. A major part of the chemicals is recovered in the flash and heat exchangers, evaporators, soda recovery unit and lime sludge reburning kiln, while the liquor is used to produce steam. The washed sulphate pulp is then transported to the paper mill where it is formed, dewatered, pressed, dried, reeled and cut for delivery. Approximately 36% of the energy losses are incurred in the soda recovery unit, ~17% in the steam plant (or steam production unit), and ~34% in the paper mill. In terms of exergy losses, ~40% are incurred in the soda recovery unit, ~31% in the steam plant and ~16% in the paper mill. The sum of the energy losses and the sum of the exergy losses in these three subprocesses are the same, but the relative proportions in the two treatments vary considerably. Furthermore, seen from an energy point of view, the unutilized outflows (or waste flows) amount to 57% of the total losses as compared to an exergy loss of only 7%. It can be seen that these flows constitute a considerably smaller resource than an energy account would lead us to believe, and it may thus be concluded that an energy balance paints an incorrect picture of the process.
The steel plant produces reinforcing steel from scrap iron. The scrap iron is smelted in two electric steel furnaces and then undergoes continuous casting. The steel is subsequently cut into blanks which are then reheated in a pusher type furnace and then rolled to circular crossections with diameters from 6 to 32 mm. The major part of the energy losses is incurred in the electric steel furnaces and during continuous casting, and amounts to ~44% of the total losses. The picture of the losses is more or less the same when we look at the exergy losses. Nearly half of the exergy losses are incurred in these two subprocesses. The difference between the energy and the exergy treatments increases when we look at the unutilized flows in the process such as exhaust gases and heat. These account for about 65% of the energy losses but only for about 28% of the exergy losses. What appears as a substantial unutilized resource flow in terms of energy is thus shown to be considerably less in terms of exergy, which is mainly due to the temperature of the flows. Thus, only a minor improvement in efficiency can be achieved by utilizing the existing unutilized flows. It is only by introducing new and more efficient processes that major improvements can be achieved.
As a comparison to the industrial processes, the study also describes the Swedish space heating system. This comparison reveals many interesting differences. The Swedish space heating system represents the largest single exergy saving potential in the country. The efficiency in the conversion to heat is estimated to be about 5% for Sweden as a whole. Two observations can be made: (1) current-day systems are highly inefficient, and (2) new technology offers enormous potential for improving Sweden's space heating system. Modern nuclear energy technology, which utilizes only a small fraction of the exergy content of nuclear fuel yields an extremely low over-all efficiency for space heating.
Finally, the study provides a short description of the price of several of the most common energy forms in relation to energy and exergy content. This is relevant since the design of the energy system mostly depends on the price of different energy forms. The approximate energy price of electricity in SEK/GJ is about 70, the price of petrol about 110, of fuel oil about 50, and of wood about 20, and district heat about 60. The high price of gasoline can be justified by its special area of use, the low price of wood can be explained by the fact that efficient energy conversion from wood is expensive. In terms of exergy, we obtain instead the following prices. (SEK/GJ): electricity about 70, gasoline about 120, fuel oil about 50, wood about 20, and district heat about 340. The very high exergy price of district heat is difficult to justify but can be explained by today's rather inefficient heat production technology. This means that it will hardly be possible to maintain the price of district heat as heat pump technology develops further. In the future, when heat pumps have reached a coefficient of performance (COP) of about 5, today's relative price of district heat would be too high.
The method presented in this paper is also valuable for long-term planning of, for example, research efforts on more efficient allocation of resources since it reveals the real losses.
A computer program is developed to calculate steam data on a micro-computer with the accuracy of ordinary steam tables. Earlier, this had only been done on large computers. Also, a simple computer based method is presented for calculating the exergy of substances.
For several years Myron Tribus and Yehia M. El-Sayed (1983) at the Center for Advanced Engineering Study, M.I.T., have been developing a method which they call "Thermoeconomics", to optimize the cost under prevailing thermodynamic conditions. The method has been applied with great success to industrial processes in the processing industry. The purpose of thermoeconomics is to improve analyses of systems by introducing ways of concurrently suggesting improvements to the analyzed system. One way in which Tribus justifies the method is as follows:
"It is much more important to be able to survey the set of possible systems approximately than to examine the wrong system exactly. It is better to be approximately right than precisely wrong."
The starting point is to consider a system surrounded by both a physical environment and an economic environment, see Fig. 6.1. The physical environment is described in terms of pressure, temperature and the chemical potentials of the substances involved. The economic environment is described in terms of the prices of the goods in question and the interest on loans.
The two environments are interconnected via cost relationships describing how the costs depend on physical quantities.
The method can be described briefly as follows:
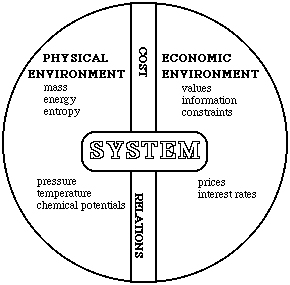
The initial stages of this working method are obviously self-explanatory and generally accepted. The most important improvements is the introduction of the environment and its effects on the process. The concept of exergy which can then be applied makes it possible, among other things, to calculate the technical losses in the system, item 7.
An engineer designing a system is expected to aim for the highest possible technical efficiency at minimum cost under the prevailing technical, economic and legal conditions (sometimes also with regard to ethical, ecological and social consequences). Scope for the following should be taken into account when doing this work:
Thermoeconomics is a method of analysis that makes this work a great deal easier.
Paper IV a, Thermoeconomic Optimization of a Heat Pump System, is an application of thermoeconomics to a single stage heat pump, which gives unexpected and interesting results. The heat pump is assumed to be made up of a compressor, a condenser, an expansion valve, an evaporator and an electric motor, i.e., a very simple assumption. The refrigerant is R12, and the heat transfer medium to the environment in the condenser and evaporator is water. The free decision variables to be chosen optimally are the efficiencies of the compressor, the condenser, the evaporator and the electric motor. The system is completely determined except as far as these variables are concerned.
The aim is to minimize the total cost of the system for a given heat production. This cost is made up partly of a running cost (electricity) and partly of costs for investment of each component. The running cost increases if the investments decrease and vice versa.
In this example, the values of the dimensioning parameters have been assumed to be: heat output produced 6 500 W (energy), running time of 5 000 hours per year, electricity cost SEK 0.25/kWh, temperature of the produced heat 60°C and temperature of the heat source 10°C. An arbitrarily operating system with all four efficiencies at 70% is assumed as starting point. The calculated total cost will then be SEK 4 221/yr, SEK 3 617/yr of which is for electricity. Optimization now gives the following efficiencies instead: compressor 0.80, condenser 0.83, evaporator 0.73, and electric motor 0.91. The total cost will now be SEK 3 388/yr instead, SEK 2 416 of which is for electricity. So by increasing the investment cost from SEK 604/yr to SEK 972/yr we make a total saving of SEK 833/yr as compared with the assumed system. At the same time the exergy losses are approximately halved from 1 933 W to 979 W, i.e., by 954 W. It is the improvements in the electric motor that account for the largest single exergy saving, so that the optimization saves us both money and exergy. It has been assumed that the electric motor would cost three times as much if its efficiency could be raised from 70 to 91%, a perfectly realistic target. It could, however, even cost nine times as much and still be profitable compared with the assumed system. It may also be added that the coefficient of performance (COP) increases from 2.25 for the assumed system to 3.36 for the optimal system.
Since the choice of the optimum system is influenced by variations in the dimensioning parameters these should also be analyzed. One is the condenser temperature, i.e., the temperature of the heat produced. The total cost is doubled from 2 336 at 40°C to 4 680 at 75°C. The energy output is the same, i.e., 6 500 W, but the exergy output changes from 434 to 713 W, which provides a better explanation of the increase in cost. When the temperature increases from 40 to 75°C, the efficiency requirements for the system as a whole become stricter, but not necessarily for each component. This is clearly shown in this case. In the context of a total increase in component costs, therefore, it is more economical to choose a cheaper evaporator. The reason is simply that an investment gives a better return in other parts of the system. The method does show where an investment is most worthwhile.
Many other relationships can be illustrated in the same way. Other refrigerants or cost relations can be assumed and the heat pump can be modified. The physical treatment of the refrigerant and the optimization of the system is made by specially developed computer programs. These are enclosed in Paper IV b, as they may be easily adapted to other refrigerants as well as other processes. The purpose of this study has been to illustrate the thermoeconomic method by applying it to a heat pump process. The exact results are therefore secondary to the presentation and discussion of the suitability of the method. However, this method for improving technical systems can never replace long practical experience or high technical expertise, but it can be a useful complementary tool to them.
Though this method is principally simple, it is difficult to apply to real processes. The purpose of this investigation, therefore, is to show the importance of applying it to technical systems and how some of the difficulties are avoided.
Ahern, J. E., 1980, The Exergy Method of Energy Systems Analysis, Wiley , N.Y.
Alfvén, H., 1975, "Exergy report may create a new energy policy", Article published in the newspaper Svenska Dagladet, Sweden, November 18, 1975.
Andersson, L., Fredriksson, R., Ljung, L., Söderström, M., and Wall, G., 1981, "Energy Quality", Report on project 80-3085, National Swedish Board for Technical Development, Stockholm. (In Swedish.)
Ayres, R. U., 1978, Resources, Environment, and Economics, Wiley, New York.
Baehr, H. D., 1965, Energie und Exergie, VDI-Verlag, Düsseldorf.
Bell, D. E., Keeney, R. L., and Raiffa, H., 1977, Conflicting Objectives in Decisions, no. 1 in Wiley IIASA International Series on Applied Systems Analysis.
Bennet, R. J. and Chorley, R. J., 1978, Environmental Systems: Philosophy, Analysis, and Control, Methuen, London.
Bennet, C. H. and Landauer, R., 1985, "The Fundamental Physical Limits of Computation", Scientific American, July 1985.
Carnot, N. L. S., 1824, Réflections sur la puissance motrice du feu et sur les machines propres a développer cette puissance, Bachelier, Paris, 1824, Fox, R., ed., Libraire Philosophique J. Vrin, Paris 1978.
Chaitin, G. J., "Toward a mathematical definition of 'life'", in: Levine, R. D., and Tribus, M., eds., The Maximum Entropy Formalism, MIT Press, Cambridge, Mass., 1979, pp. 477-498.
Chapman, P. F. and Roberts, F., 1983, Metal Resources and Energy, Butterworths, London.
Edgerton, R. H., 1982, Available Energy and Environmental Economics, Lexington Books.
Egnéus, H., Eriksson, B., Ivarsson, C.-G., Jungen, B., Keen, K., and Lindeberg, S., "Det glada tjugotalet" ("The happy twenties"), Report from the project "Swedens future energy provision in a historical and global perspective", Centre for Interdisciplinary Studies of Human Conditions, University of Göteborg. (In Swedish)
El-Sayed, Y. M. and Tribus, M., 1983, "Strategic use of thermoeconomics for systems improvement", in R. A. Gaggioli ed., Efficiency and Costing, ACS Symposium Series no. 235.
Eriksson, K.-E., 1982 a, "Exergetics", Report no. 82-2, Physical Resource Theory Group, Chalmers University of Technology and University of Göteborg, Sweden.
Eriksson, K.-E., 1982 b, "Thermodynamical Aspects on Ecology/Economics", in: Jansson, A.-M., ed., Integration of Economy and Ecology: An Outlook for the Eighties, Wallenberg Symposium, Stockholm 1984, pp. 39-45.
Eriksson, B., Eriksson, K.-E., Olsson, K., and Wall, G., 1975, "Exergy the 'Useful Energy'", Article published in the newspaper Svenska Dagladet, Sweden, December 6, 1975.
Eriksson, B., Eriksson, K.-E., Olsson, K., and Wall, G., 1976, "Towards an Integrated Accounting of Energy and Other Natural Resources", Report no. 75-33, Institute of Theoretical Physics, Chalmers University of Technology and University of Göteborg, Sweden.
Eriksson, B., Eriksson, K.-E., and Wall, G., 1978, "Basic Thermodynamics of Energy Conversions and Energy Use", Report no. 77-41, Institute of Theoretical Physics, Chalmers University of Technology and University of Göteborg, Sweden.
Eriksson, K.-E., Islam, S., and Skagerstam, B.-S., 1982, Nature, vol. 296, pp. 540-542.
Eriksson, K.-E., Islam, S., and Tengström, E., 1981, "Resources in Nature and Society: Suggestions for a general theoretical basis and a consistent conceptual framework", Report no. 81-8, Physical Resource Theory Group, Chalmers University of Technology and University of Göteborg, Sweden.
Eriksson, K.-E., Islam, S., Karlsson, S., Månsson, B., Peressutti, G., and Wall, G., 1981, "Physical Resource Theory", Report no. 81-11, Physical Resource Theory Group, Chalmers University of Technology and University of Göteborg, Sweden.
Eriksson, K.-E. and Lindgren, K., 1986, "Structural Information in Self-organizing Systems", Report no. 86-1, Physical Resource Theory Group, Chalmers University of Technology and University of Göteborg, Sweden. (To appear in Physica Scripta.)
Eriksson, K.-E. and Kåberger, T., 1984 revised 1986, "Flow-based Measures of System Evolution", Report no. 86-2, Physical Resource Theory Group, Chalmers University of Technology and University of Göteborg, Sweden.
Evans, R. B., 1969, A proof that essergy is the only consistent measure of potential work, Thesis, Dartmouth College, Hanover, New Hampshire.
Gaggioli, R. A., ed., 1980, Thermodynamics: Second Law Analysis, ACS Symposium Series no. 122, American Chemical Society, Washington, D.C.
Gaggioli, R. A., ed., 1983, Efficiency and Costing, ACS Symposium Series no. 235, American Chemical Society, Washington, D.C.
Gibbs, J. W., 1873, Collected Works, Yale University Press, New Haven 1948. Originally published in Trans. Conn. Acad., Vol. 2, pp. 382-404.
Gilliland, M. W., ed., 1978, Energy Analysis: A New Public Policy Tool, Westview Press, Boulder, Colorado.
Grubbström, R. W., 1980, "Towards a Theoretical Basis for Energy Economics", Technical Report NPS-54-80-015 Naval Postgraduate School, Monterey, California 93940.
Haken, H., ed., 1980, Synergetic systems, Springer-Verlag, Berlin.
Haken, H., 1983, Synergetics, 3rd ed., Springer-Verlag, Berlin.
Haken, H., 1984, Advanced Synergetics, Springer-Verlag, Berlin.
Johansson, T. B. and Lönnroth, M., 1975, "Energy analysis - an introduction" ("Energianalys - en introduktion"), Report no. 403 from the group Energy and Society at the Secretariat of Futurological Studies (Energi och Samhälle, in Swedish), Stockholm.
Karlsson, S., 1982 a, "The Exergy of Incoherent Electromagnetic Radiation", Physica Scripta, vol. 26, pp. 329-332.
Karlsson, S., 1982 b, "Exergin i US Standard Atmosphere 1976", Report no. 82-14, Physical Resource Theory Group, Chalmers University of Technology and University of Göteborg, Sweden. (In Swedish.)
Macrakis, M. S., ed., 1974, Energy, MIT Press, Cambridge, Mass.
Meadows, D. H., Meadows, D. L., Randers, J., and Behrens III, W. W., 1972, The Limits to Growth, Universe Books, New York.
Meadows, D. H., ed., 1977, Alternatives to Growth, Ballinger, Cambridge, Mass.
Moran, M. J., 1982, Availability Analysis: A Guide to Efficient Energy Use, Prentice-Hall, Englewood Cliffs, New Jersey.
Månsson, B. Å. G., 1981, "Exergy and equations of state for fluids", Report no. 81-10, Physical Resource Theory Group, Chalmers University of Technology and University of Göteborg, Sweden.
Månsson, B. Å. G., 1985 a, "Entropy Production in Oscillating Chemical Systems", Z. Naturforsch., vol. 40 a, pp. 877-884.
Månsson, B. Å. G., 1985 b, Contributions to Physical Resource Theory, PhD Thesis, Chalmers University of Technology, Göteborg.
Penner, S., ed., 1980, "Second Law Analysis of Energy Devices and Processes", Energy, vol. 5, pp. 665-1011.
Prigogine, I., 1980, From Being to Becoming. Time and complexity in the physical science, W. H. Freeman and Co., Oxford.
Rant, Z., 1956, Forschung Ing.-Wesens, vol. 22, no. 36.
Tengström, E., 1985, Human Ecology - A New Discipline? A Short Tentative Description of the Institutional and Intellectual History of Human Ecology, Göteborg.
Thomas, J. A. G., ed., 1977, Energy Analysis, Westview Press, Boulder, Colorado.
Wall, G., 1978 a, "Energy Accounting with Exergy", VVS Special, no. 1, pp. 8-11, Förlags AB VVS, Stockholm. (In Swedish.)
Wall, G., 1978 b, "The use of energy and other natural resources in a society", paper presented at the 1st International Conference on Energy and Community Development, Athens, Greece, July 10-15, 1978.
Wall, G., 1981, "Exergy Conversion in Swedish Society", Report no. 80-1, Physical Resource Theory Group, Chalmers University of Technology and University of Göteborg, Sweden.
Wall, G., 1982, "Compendium in Natural Resources and Society, part A", Chapter 5, Physical Resource Theory Group, Chalmers University of Technology and University of Göteborg, Sweden. (In Swedish)
Wall, G., 1983 a, "Energy and Exergy Flows in Industrial Processes", Report no. 83-11, Physical Resource Theory Group, Chalmers University of Technology and University of Göteborg, Sweden. (In Swedish.)
Wall, G., 1983 b, "Energy and Material Conversion in Sweden", Energy Technology, no. 1, 1983, National Swedish Board for Technical development, Stockholm, pp. 3-5, also available in swedish: "Den svenska energi- och materialomsättningen", Energiteknik, no. 3, 1982, Styrelsen för teknisk utveckling, Stockholm, pp. 24-26.
Wall, G., 1985, "Thermoeconomic Optimization of a Heat Pump System", Report no. 85-5, Physical Resource Theory Group, Chalmers University of Technology and University of Göteborg, Sweden. (To appear in Energy.)
Wall, G., 1986, "Thermoeconomic Optimization of a Heat Pump System", Energy Technology, no. 2, 1986, National Swedish Board for Technical Development, Stockholm, pp. 3-6, also available in swedish: "Termoekonomisk optimering av en värmepumpprocess", Energiteknik, no. 1, 1986, Styrelsen för teknisk utveckling, Stockholm, pp. 3-6.
Wall, G. ed., 1981, "Symposium on Concepts of Energy Quality", Proceedings of Symposium on Concepts of Energy Quality at Chalmers University of Technology, Göteborg, Sweden, November 18, 1981, Physical Resource Theory Group, Chalmers University of Technology and University of Göteborg, Sweden. (Partly in Swedish.)